Ophthalmologists striving to improve outcomes for nAMD patients are set to gain greater insight with the release of deepeye Medical AI therapy planning support
Heidelberg, Germany (5 March 2025) – A new level of insight to therapy planning for nAMD patients is expected with Heidelberg Engineering and deepeye Medical’s collaboration, following three years of collaborative development.

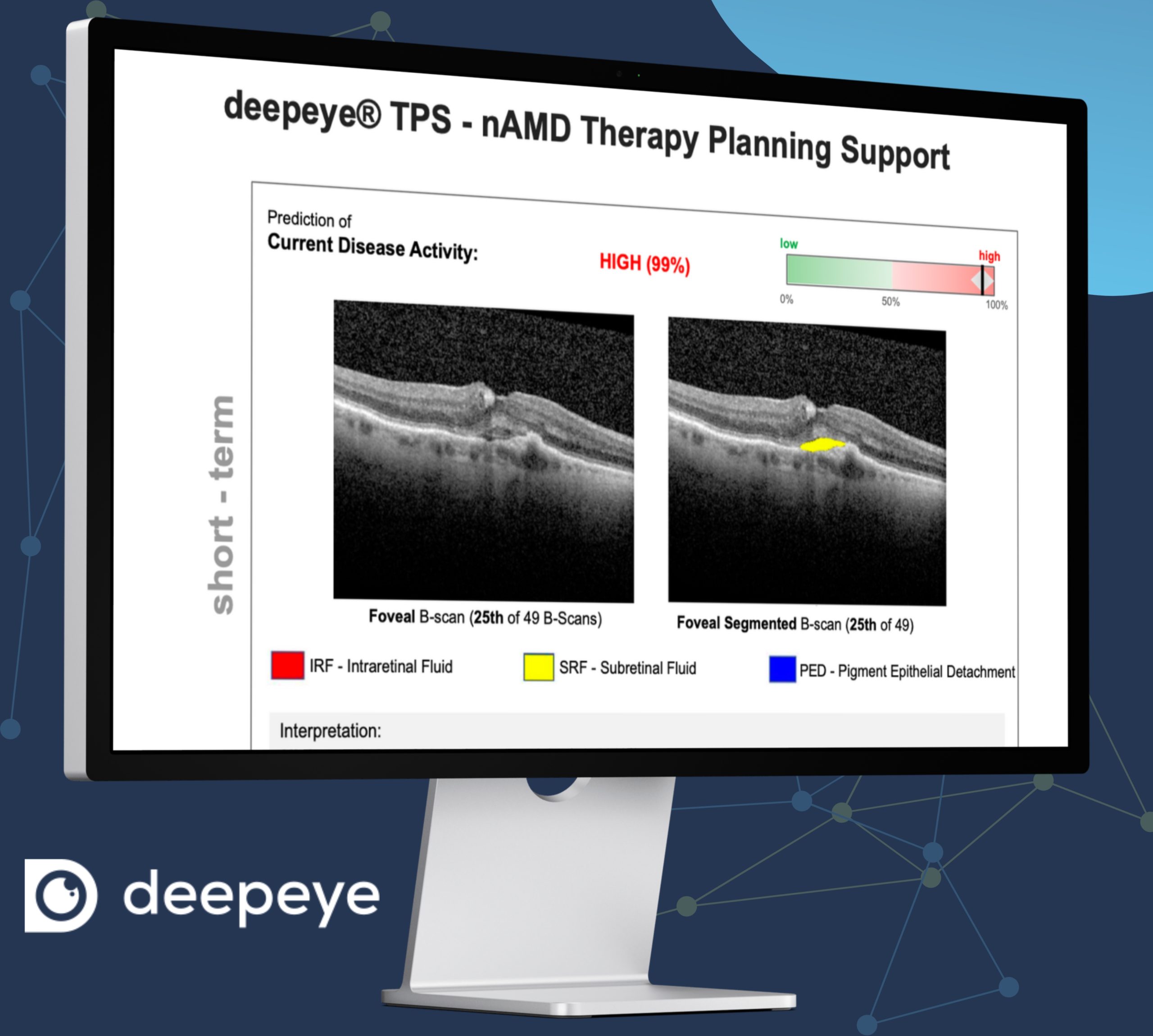
deepeye® TPS’s¹ (Therapy Planning Support) for anti-VEGF therapy is expected to achieve full CE marking this spring, following a year of beta testing and various successful collaborations with research partners, including Heidelberg Engineering, Bayer, Novartis, and Roche.
“We have been testing deepeye® TPS in practice for a year with several hundred cases. The seamless integration with HEYEX 2 saves us time in OCT assessment and case documentation. For our tele-ophthalmic network (IVI portal) with over 60 practices, it was crucial that the AI assistant supports all OCT device manufacturers. With the approval as a medical device, we will soon be able to fully exploit the advantages of deepeye. Personally, I particularly appreciate the possibility of informing patients and relatives more easily about the expected treatment burden thanks to visualization and initial therapy prognosis, thus reducing the associated stress and improving adherence” says Prof. Dr. Albrecht Lommatzsch, Co-owner of the Eye Center at St. Franziskus-Hospital Münster, 2nd Chairman of the German Retina Society
Developed from insights gathered from more than one million retinal images from thousands of patients across 200 centers during a ten-year period, deepeye TPS¹ is designed to assist eye care specialists in therapy planning for patients with nAMD and has the potential to optimize nAMD management strategies, leading to more informed treatment decisions.
 deepeye Medical emerged from one of the world’s first tele-ophthalmic networks (IVI Portal Franziskus Münster), where expert reviews of other ophthalmologists’ OCT readings tripled outcomes (3x VA) and doubled retention (2x persistence) compared to other real-world studies.
deepeye Medical emerged from one of the world’s first tele-ophthalmic networks (IVI Portal Franziskus Münster), where expert reviews of other ophthalmologists’ OCT readings tripled outcomes (3x VA) and doubled retention (2x persistence) compared to other real-world studies.
“Our AI mimics this proven clinical method of double reading that is widely established in radiology or mammography to empower all ophthalmologists to personalize treatment faster, driving outcomes and adherence. With Heidelberg AppWay we are set to empower retina specialists with data-driven insights to optimize therapy planning. Better patient outcomes and economically viable practices are the objective” added Manuel Opitz, CEO of deepeye Medical.
“Heidelberg Engineering are pleased to include the deepeye TPS application as our latest addition to our Heidelberg AppWay AI marketplace. This deepeye TPS solution will provide our current SPECTRALIS AND HEYEX 2 users with a tool to aid decision making in when providing anti-angiogenic therapies to their nAMD patients. This new addition to the Heidelberg Engineering AppWay marketplace further reflects our ongoing commitment to delivering innovative AI solutions that enhance patient care and streamline clinical workflows.” says Kfir Azoulay from Heidelberg Engineering.
Accessible via Heidelberg AppWay, this innovative solution will be showcased at the AAD Conference in Düsseldorf (March 19-22) at Stand #512. Early users of deepeye will actively participate in key discussions, including the Heidelberg AppWay Symposium on March 21 and the Expert Panel Discussion at the Heidelberg booth (#221) on March 22, offering valuable insights into the future of AI-driven therapy planning.
¹deepeye TPS is currently in the process of obtaining CE marking. This product is a medical device and may have potential risks as a treatment planning support for patients with nAMD. For detailed safety information, please refer to the electronic instructions for use (eIFU).


